Ketosis in Cattle: Symptoms, Treatment, and Prevention Guide
Definition and Etiology of Ketosis in Cattle
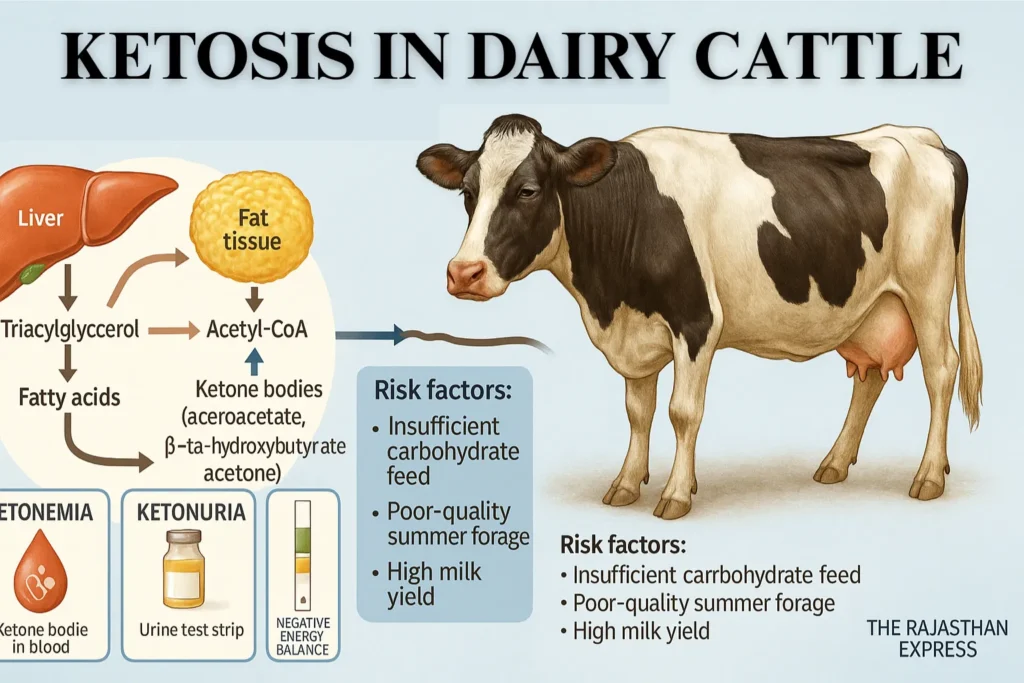
Ketosis is a multifactorial energy metabolism-related disease, commonly observed in high-yielding dairy animals after parturition (especially within the first month).
In this disease, blood glucose levels decrease, primarily due to inadequate carbohydrate-rich feed (such as grains) provided to the animal.
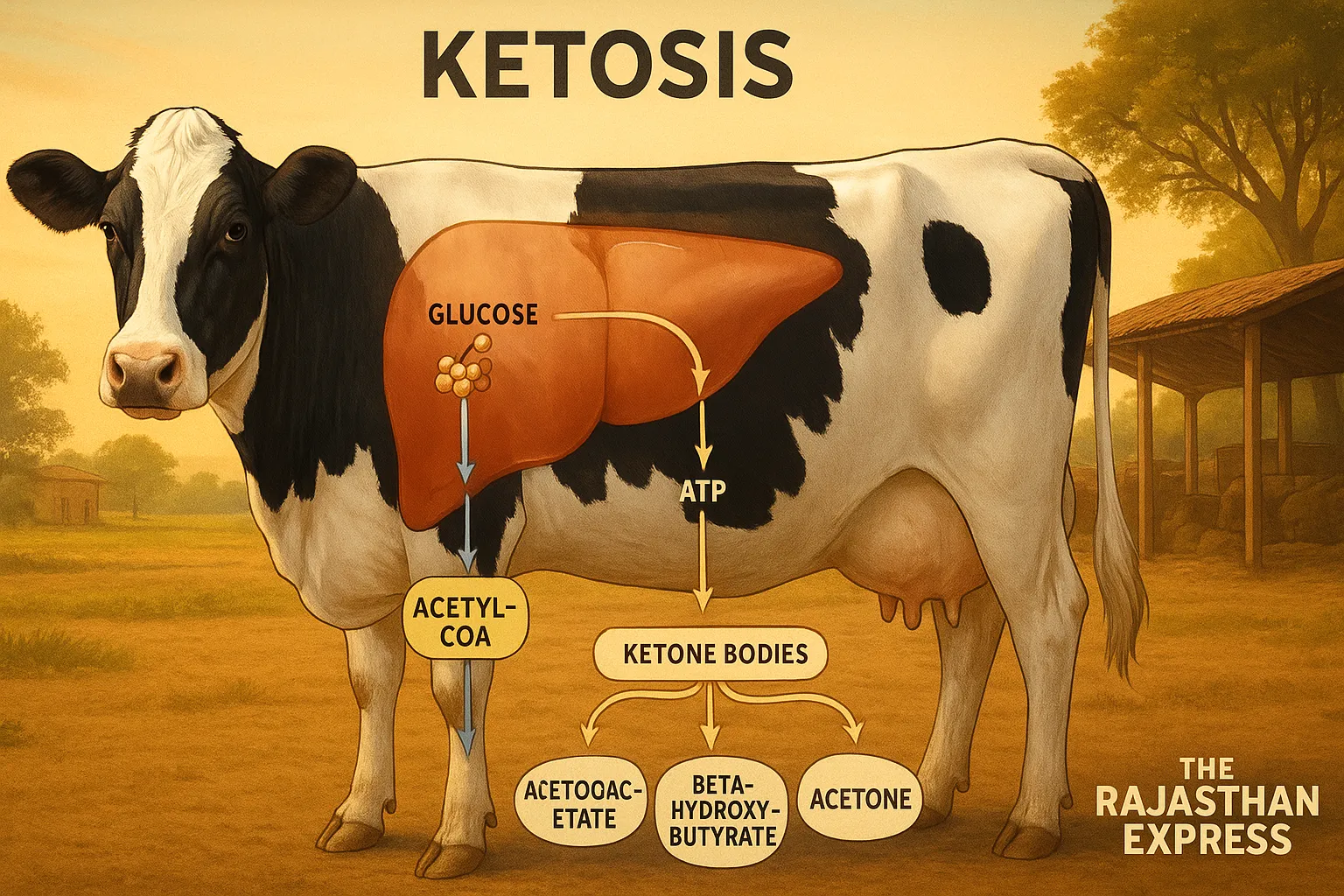
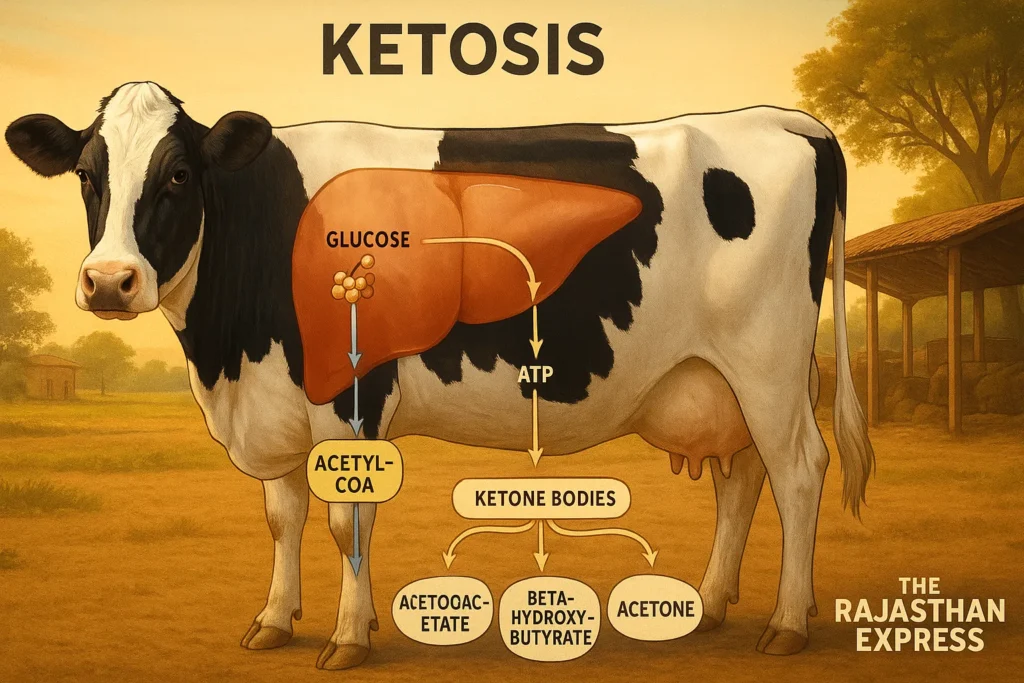
Glucose, a hexose monosaccharide sugar, is the primary energy source in the body. It enters the mitochondria of cells to produce ATP (adenosine triphosphate), the energy molecule.
Carbohydrates are stored as glycogen in animals. When food is unavailable, glycogen converts to glucose via glycogenolysis to provide energy, mainly occurring in the liver.
Similarly, excess fat is stored as triacylglycerol (TAG). During hunger, fasting, or insufficient carbohydrate intake, this fat breaks down into fatty acids via lipolysis for energy. These fatty acids further convert to Acetyl-CoA.
One part of Acetyl-CoA enters the Krebs cycle to produce energy, while the other converts into ketone bodies (acetoacetate, beta-hydroxybutyrate, and acetone).
Ketosis in Cattle
| Alternative Names | Acetonemia, Ketonemia, Hyperketonemia, Bovine Ketosis, Subclinical Ketosis (SCK) |
|---|---|
| Definition | A metabolic disorder of high-producing dairy cattle, primarily in early lactation, caused by negative energy balance leading to excess mobilization of body fat and accumulation of ketone bodies in blood, milk, and urine. |
| Etiology | Inadequate dietary energy intake post-calving; excessive glucose demand for lactose synthesis; hormonal shifts reducing gluconeogenesis; excessive lipid mobilization from adipose tissue. |
| Pathophysiology | Reduced rumen propionate → depletion of liver glycogen → mobilization of NEFAs → hepatic conversion to ketone bodies (β-hydroxybutyrate, acetoacetate, acetone) → hypoglycemia, ketosis, metabolic depression. |
| Risk Factors |
|
| Forms |
|
| Prevalence | Clinical: 2–15% of fresh cows; Subclinical: 40–60% within 2–3 weeks postpartum (USDA-ARS, NIFA, Merck Vet Manual). |
| Clinical Signs |
|
| Diagnosis |
|
| Treatment |
|
| Advanced/Research-based Interventions |
|
| Prevention |
|
| Economic Impact | Per case: US$256–375 in lost milk, reproduction delays, culling risk; higher costs with concurrent diseases. |
| References | USDA-ARS Research Reports; Merck Veterinary Manual; Penn State Extension; University of Wisconsin Dairy Extension; National Institute of Food and Agriculture (NIFA); University of Maryland Extension. , The Rajasthan Express |
Adverse effects of excess ketone bodies:
- Ketonemia: Presence of ketone bodies in blood
- Ketonuria: Presence of ketone bodies in urine
The coexistence of both conditions is termed ketosis.
Metabolic Disease in Animals: Definition, Types, and Key Characteristics
Definition:
Metabolic Diseases are those diseases which occur due to deficiency, imbalance of nutrients, or disturbance of metabolism in the body. These diseases are especially related to milk production, pregnancy, and the energy requirements of the body.
Read More About : Metabolic Disease
Main Features of Metabolic Disease in Dairy Cattle
- These diseases are Non-Contagious — do not spread from one animal to another.
- In most cases, these diseases are related to the production system, hence they are also called Production Diseases.
- Especially pregnant and milk-producing animals (like cow and buffalo) are more susceptible to these diseases.
- The possibility of metabolic diseases increases with increasing milk production.
- In native cows (Zebu cattle), the highest possibility of metabolic disease is found in the third calving.
- In exotic cows (Exotic Cattle), this possibility is highest in the fifth calving.
- In buffaloes, the possibility of metabolic diseases is mostly in the fourth calving.
- The order of possibility of metabolic diseases is as follows:
Exotic Cow > Native Cow > Buffalo - The possibility of Downer Cow Syndrome, Ketosis, Postpartum Haemoglobinuria, and Mastitis disease is higher in exotic cows, especially in the Holstein-Friesian breed.
- These same diseases are found mostly in the Sahiwal breed in native cows.
- The possibility of Milk Fever is highest in Jersey cows.
We have also explained these diseases in detail.
Explore the key metabolic diseases in cattle with quick links to each section:
- Milk Fever – Calcium deficiency immediately after calving
- Downer Cow Syndrome – Complications from prolonged lying after milk fever
- Ketosis In Cattle – Energy deficiency and ketone accumulation
- Postparturient Haemoglobinuria – Red blood cell destruction due to phosphorus deficiency
- Grass Tetany – Muscle cramps from magnesium deficiency
- Pregnancy Toxemia – Energy deficiency in late pregnancy
Negative Energy Balance (NEB)
Ketosis primarily results from Negative Energy Balance (NEB), involving glucose deficiency, ketonemia, and ketonuria. This state arises due to disruptions in fat and carbohydrate metabolism.
In summers, when cattle receive low-quality/quantity fodder, stored fat breaks down, producing ketone bodies excreted via blood and urine.
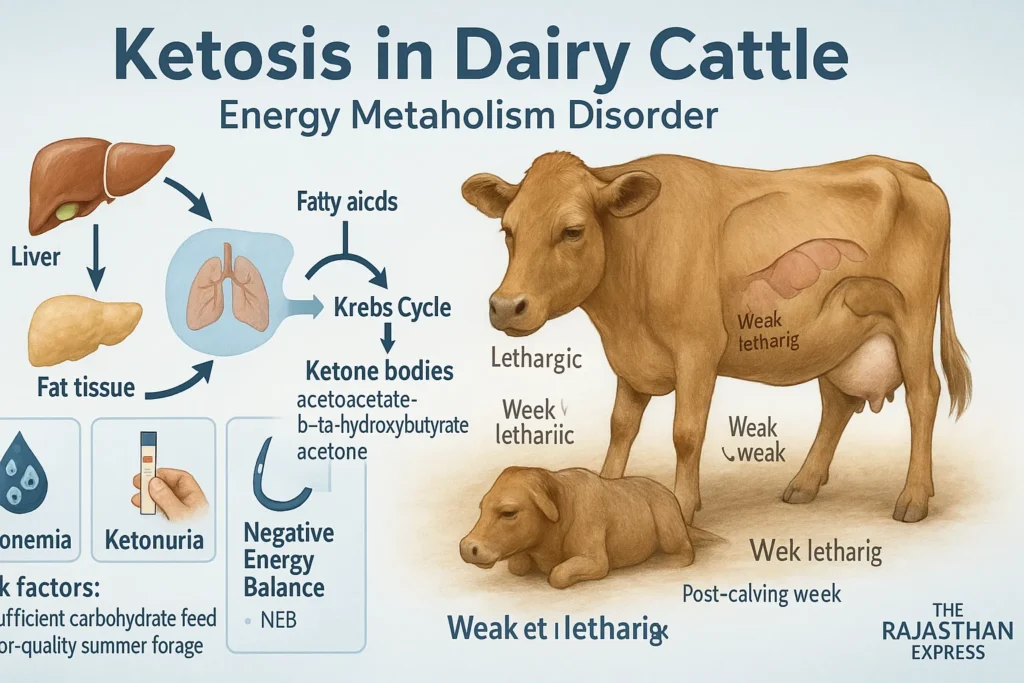
Causes of Ketosis in Cattle
1. Nutritional Causes
- Fasting or anorexia
- Low-carbohydrate diet
- Excessive protein intake
- Overuse of silage
📝 Note: Excessive protein fails to meet energy needs as surplus protein isn’t stored like carbohydrates and is excreted via urine. A protein-only diet increases ketosis risk.
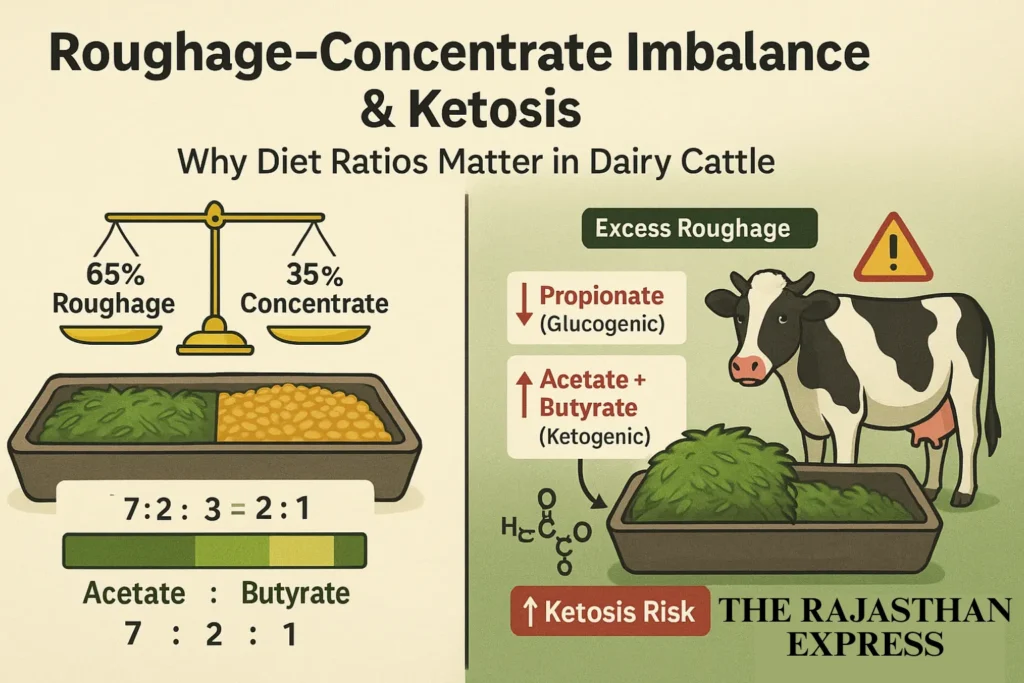
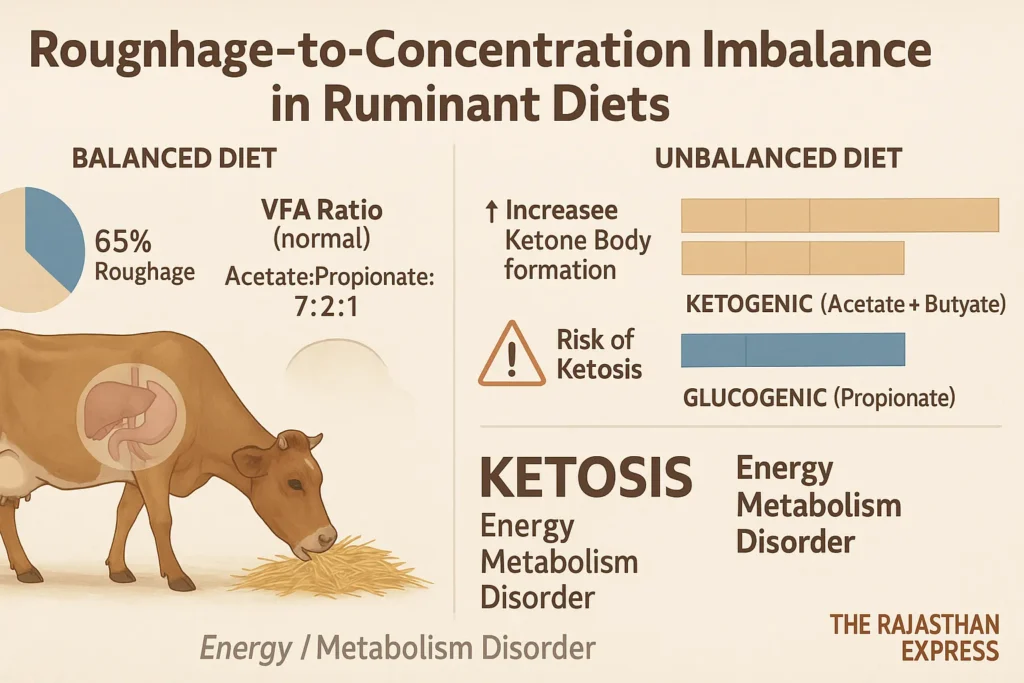
2. Roughage-Concentrate Imbalance
Ideal diet ratio for ruminants (e.g., cows/buffaloes): 65% roughage : 35% concentrate.
Imbalance (e.g., excess roughage, low concentrate) elevates ketosis risk due to VFAs (Volatile Fatty Acids) imbalance:
- Balanced VFA ratio: Acetate : Propionate : Butyrate = 7:2:1
- *Ketogenic (Acetate + Butyrate) : Glucogenic (Propionate) = 4:1*
- Propionate is glucogenic (produces glucose).
- Acetate/Butyrate are ketogenic (produce ketone bodies).
📌 Summary: Excess roughage reduces propionate, increasing ketone bodies and ketosis risk.
3. Animal-Related Causes
- Lactose loss via milk (~45g/kg milk) in high-yielding cattle, causing energy deficiency and reduced liver efficiency.
- Lactose is a carbohydrate sugar in milk.
4. Hormonal Causes
- Reduced glucocorticoids or gluconeogenesis during stressful events (e.g., pregnancy/parturition).
- Rare Cause: Insulin deficiency-related ketosis.
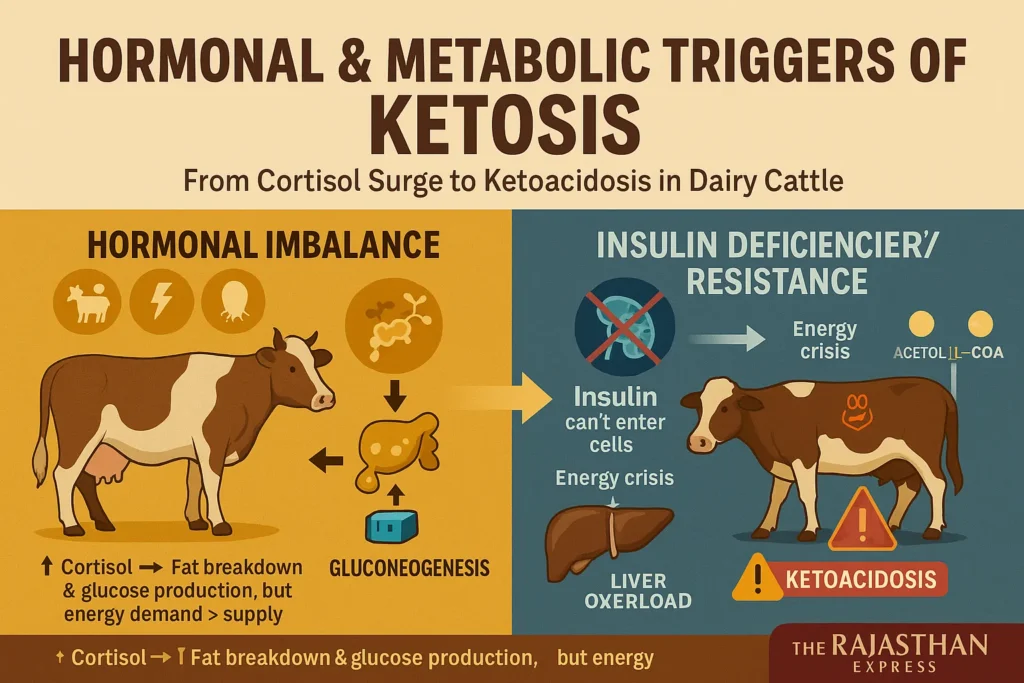
Hormonal Imbalance:
During pregnancy, parturition, or stress, elevated glucocorticoid hormones (e.g., cortisol) accelerate fat breakdown and gluconeogenesis. However, this response often fails to meet energy demands due to limited gluconeogenesis capacity.
Insulin Deficiency/Resistance (Rare Cause):
When insulin is deficient or cells become insulin-resistant, blood glucose cannot enter cells effectively. This causes:
- Hyperglycemia (Note: Classic in Type 1 DM; ruminant ketosis typically shows hypoglycemia).
- Cellular energy crisis → Triggers breakdown of stored fat (triacylglycerol) and glycogen.
- Fatty acid beta-oxidation → Produces excess Acetyl-CoA.
- Liver overload → Surplus Acetyl-CoA converts to ketone bodies (acetoacetate, beta-hydroxybutyrate, acetone).
- Ketoacidosis: Excessive ketones cause metabolic acidosis, severely disrupting acid-base balance.
5. Predisposing Factors
- Common in high-yielding crossbred stall-fed cows/buffaloes.
- Metabolic diseases frequently occur in:
- Indigenous cows: 3rd lactation
- Buffaloes: 4th lactation
- Exotic cows: 5th lactation
- Ketosis is most prevalent in the first month post-parturition.


Symptoms of Ketosis in Cattle
1. Wasting Form / Digestive Form (Most Common)
⚠️ Key Symptoms:
- Animal eats grass/hay but completely refuses grains.
- Later reduces total feed/water intake.
- May chew inedible objects (Pica) due to mineral/energy deficiency.
- Milk production declines.
- Vinegar/rotten fruit-like odor in breath/urine (due to ketonemia/ketonuria).
- Weight loss, dehydration, emaciated appearance.
- Depression, abdominal pain, “hangdog appearance.”
ℹ️ Note: Non-fatal; recovery possible in 1–2 months with timely energy-balanced diet.

2. Nervous Form (Less Common, Severe)
⚠️ Key Symptoms:
- Circling with crossed legs (hallmark sign).
- Head pressing against walls.
- Blind-like stumbling gait.
- Compulsive licking/chewing of objects.
- Tetanus-like symptoms:
- Hyperesthesia (hypersensitivity)
- Loss of balance, frequent falls.
- Symptoms appear intermittently (every 8–10 hours).
Diagnosis of Ketosis in Cattle
Clinical History and Symptoms
Based on feed aversion, weight loss, reduced milk yield, and breath/urine odor.
1. Laboratory Tests
- Rothera’s Test: Detects ketone bodies in urine/milk. Pink/purple color indicates ketones.
- Confirm hypoglycemia, ketonemia, and ketonuria.
- Milk analysis during NEB:
- ↑ Fat content
- ↓ Protein content
Samples for Testing: Blood, urine, milk.

2. Differential Diagnosis
(1) For Wasting Form
Rule out: Traumatic reticulitis, pericarditis, diaphragmatic hernia, vagal indigestion, pneumonia, metritis, cystitis, pyelonephritis, displaced abomasum.
(2) For Nervous Form
Rule out: Tetanus, rabies, encephalitis, lead poisoning, lactation tetany, hypomagnesemia.
Treatment for Ketosis in Cattle
1. Replacement Therapy
Goal: Restore glucose, halt ketone production.
- 25% Dextrose (500–1000 ml, IV, once daily, 2–3 days)
- Propylene glycol (225g orally, 3–5 days)
- Sodium propionate (110–225g orally, 3–6 days)
- Jaggery (natural carbohydrate source)
2. Hormonal Therapy
- Dexamethasone sodium phosphate (40 mg, IV, 4–6 days) + Glucose infusion
- Long-acting insulin (200–300 units, SC)
3. Supportive Therapy
- Vitamin B1/B-complex (10 ml, IM, alternate days)
- Phosphorus/cobalt-enriched mineral mixtures.
Prevention of Ketosis in Cattle
1. Nutritional Management
- Avoid fasting/overfeeding during parturition.
- Maintain diet ratio: 65% roughage : 35% concentrate.
2. Late Pregnancy Ration (Additional to Maintenance Feed)
| Late Pregnancy Ration (Additional to Maintenance Feed) | |
| Animal | Extra Concentrate |
|---|---|
| Indigenous Cow | 1.25 kg |
| Buffalo / Exotic Cow | 1.75 kg |
| Sheep / Goat | 250 g |
| Late Pregnancy Ration (Additional to Maintenance Feed) | |
3. Lactation Management
- Late Lactation: Increase energy via digestible fiber; reduce starch.
- Early Lactation: Diet must contain 38–41% Non-Fiber Carbohydrates (NFC).
4. Monensin Sodium Protocol
- Monensin sodium (300 mg/animal/day) during transition period (pre/post-parturition).
- Prevents subclinical/non-infectious ketosis.






⚠️ Is your cattle losing weight or circling? Learn ketosis symptoms (wasting/nervous forms), IV dextrose treatment, prevention diet ratios & PDF download. Vet-approved 2025 strategies!
THE RAJASTHAN EXPRESS
Frequently Asked Questions About Ketosis in Dairy Cattle
Why do propylene glycol (PG) treatments sometimes fail in ketotic cattle despite correct dosing?
- PG works via ruminal fermentation to propionate and direct absorption for hepatic gluconeogenesis.
- Failure causes include:
- Rumen motility impairment (e.g., subclinical hypocalcemia, displaced abomasum) reducing propionate production.
- Hepatic lipidosis decreasing gluconeogenic enzyme activity and PG utilization.
- Concurrent infections (metritis, mastitis) releasing cytokines that downregulate gluconeogenesis.
- Dosing issues: effective oral dose ≈ 250–300 mL/day; excess (>500 mL/day) can cause diarrhea and microbial disruption.
Does ambient temperature significantly alter ketosis risk or PG treatment efficacy?
- Heat stress (THI > 68) reduces feed intake, increases negative energy balance, and raises NEFA levels.
- Reduced insulin sensitivity under heat stress accelerates ketogenesis.
- PG may be less effective in hot weather due to dehydration and faster rumen passage.
Can milk ketone strips reliably replace blood BHBA testing for subclinical ketosis?
- Milk ketone strips are moderately sensitive/specific and useful for quick on-farm screening.
- May miss early cases detectable in blood before day 4 postpartum.
- False negatives possible with mild SCK; best used alongside targeted blood testing for high-risk cows.
What is the economic cost of subclinical ketosis?
- Milk yield loss: typically 2–3 kg/day in early lactation.
- Reproductive delays: more days open and extra inseminations required.
- Higher secondary disease incidence: displaced abomasum, mastitis, metritis.
- Increased culling risk.
- Total cost per case: usually in the low- to mid-hundreds of dollars.
Do behavioural changes allow earlier ketosis detection than lab tests?
- Activity and rumination monitors detect changes 3–7 days before lab confirmation.
- Reduced walking and feeding time often precede diagnosis.
- Best used for early warning with follow-up BHBA or milk ketone testing.
Is there evidence for B-vitamin injections in ketosis treatment?
- Vitamin B12 supports propionate metabolism in gluconeogenesis.
- Thiamine (B1) helps counter possible rumen microbial disruption.
- Studies show they can speed recovery when used with PG, but not as a standalone therapy.
- Typical protocol: daily intramuscular injections for several days — follow vet guidance.
How do genetics and fat distribution influence ketosis risk?
- Higher visceral fat before calving increases NEFA release postpartum.
- BHB levels have moderate heritability; genes like DGAT1 and GHR affect metabolism.
- Holsteins generally more prone than Jerseys at similar output due to fat mobilization differences.
How does ketosis differ between dairy cattle and buffalo?
- Buffalo mobilize less NEFA but have higher insulin resistance than cattle.
- Ketosis diagnostic thresholds for BHB are slightly lower in buffalo.
- PG often less effective; glycerol plus IV glucose may work better.
- Buffalo are more heat-tolerant, lowering heat-related ketosis risk.
Can partial milking prevent ketosis without reducing yield?
- In high-risk, over-conditioned cows, reducing milk removal in the first few days can lower glucose drain.
- Best targeted to multiparous high-BCS cows, not first-lactation animals.
- When used short-term, long-term yield is usually unaffected.
Why do glucocorticoid treatments for ketosis sometimes cause side effects?
- Hypokalemia: Some glucocorticoids (e.g., isoflupredone) cause renal potassium loss, especially with repeated doses.
- Infection risk: High or repeated dexamethasone doses can suppress immune function.
- Use lowest effective dose, limit frequency, and monitor potassium in treated cows.