Felycin-CA1 Tablets for Cats: FDA-Approved Sirolimus Treatment for Subclinical HCM
What Are Felycin-CA1 Tablets? (Sirolimus for Cats)
Felycin®-CA1 is the first veterinary-specific brand of Sirolimus (Rapamycin). It is formulated as an enteric-coated tablet for cats, which delays release until the tablet reaches the small intestine to prevent the acid-labile drug from degrading in the stomach. This ensures consistent absorption but does not provide a sustained or gradual release; its pharmacokinetic profile is bioequivalent to other immediate-release sirolimus products. The key innovation is its small size and precise dosing for veterinary use, enabling an intermittent dosing schedule (e.g., once weekly). This pharmacodynamic strategy aims to inhibit specific cellular pathways (mTORC1) beneficial for heart disease while limiting inhibition of others (mTORC2) linked to side effects. It is specifically designed to help manage chronic conditions such as Hypertrophic Cardiomyopathy (HCM) in cats by modulating cellular growth and reducing inflammation.
Active Ingredient: Sirolimus (Rapamycin)
Sirolimus (Rapamycin) is a macrocyclic lactone produced by the bacterium Streptomyces hygroscopicus.
Felycin-CA1 Tablets (Sirolimus for Cats)
| Drug Name | Felycin®-CA1 |
|---|---|
| Generic Name | Sirolimus (Rapamycin) |
| Drug Class | mTOR Inhibitor / Immunosuppressant |
| Formulation | Delayed-release enteric-coated tablets |
| Manufacturer | PRN Pharmacal |
| FDA Status | Conditionally Approved – March 14, 2025 |
| Species | Domestic Cat (Felis catus) |
| Indication | Subclinical Hypertrophic Cardiomyopathy (HCM) |
| Diagnostic Criteria |
|
| Dosage & Administration |
|
| Tablet Strengths |
|
| Pharmacokinetics |
|
| Mechanism of Action | Inhibits mTORC1 → Reduces cardiac hypertrophy, inflammation, and oxidative stress; promotes autophagy |
| Clinical Efficacy |
|
| Side Effects (Common) |
|
| Side Effects (Cardiac) |
|
| Severe Risks |
|
| Contraindications |
|
| Genetic Consideration | Use with caution in cats with MDR1 gene mutation (↓ drug clearance) |
| Drug Interactions |
|
| Effect on Vaccines |
|
| Monitoring Protocols |
|
| Storage Instructions |
|
| Handling Precautions |
|
| Emergency Contacts |
|
| Source: The Rajasthan Express & PRN Pharmacal, 2025 | |
Felycin-CA1 Uses: Treating Subclinical HCM in Cats
Indication and FDA Approval
- Felycin®-CA1 (Sirolimus) is used to treat Subclinical Hypertrophic Cardiomyopathy (HCM) in cats.
- On March 14, 2025, the FDA granted Conditional Approval to Felycin-CA1 (Sirolimus delayed-release tablets). It is the world’s first FDA-approved drug for treating subclinical HCM in cats.
Understanding Hypertrophic Cardiomyopathy (HCM)
Hypertrophic Cardiomyopathy (HCM) is a common and serious heart disease in cats. It occurs when the muscular wall of the heart—particularly the left ventricle—becomes abnormally thickened. This thickening interferes with the heart’s ability to pump blood efficiently, potentially leading to heart failure if left untreated.
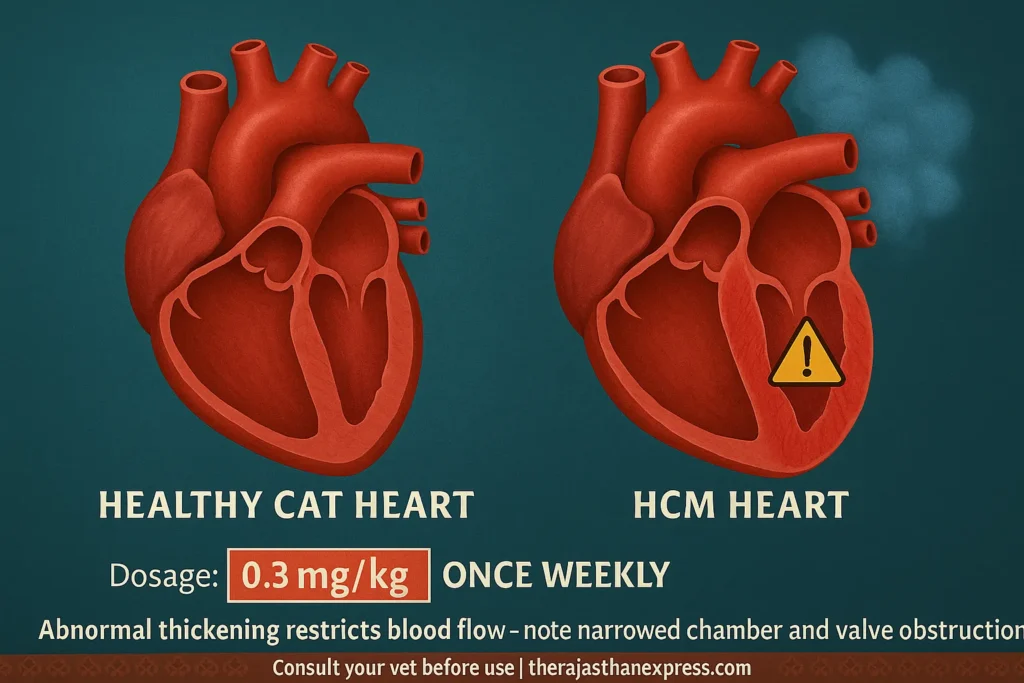
When this wall thickens:
- The heart has a harder time filling with blood.
- Blood pressure builds up in the heart and lungs.
- This can lead to breathing problems, fluid in the lungs, or even heart failure.
- In some cases, it can cause sudden death, especially if it’s not diagnosed and treated.
Some cats with HCM also have a valve problem that makes it harder for blood to flow out of the heart to the rest of the body.
Felycin-CA1 Tablet Specifications
Active Ingredient
- Sirolimus (Rapamycin)
Available Strengths & Color Coding
- 0.4 mg: Orange tablet
- 1.2 mg: Blue tablet
- 2.4 mg: White tablet
Physical Characteristics
- Enteric film-coated tablet: This special coating ensures the drug dissolves in the intestines.
Subclinical HCM in Cats: Diagnosis Criteria
What is Hypertrophic Cardiomyopathy (HCM) in Cats?
Hypertrophic Cardiomyopathy (HCM) is a common and serious heart disease in cats. It occurs when the muscular wall of the heart—particularly the left ventricle—becomes abnormally thickened. This thickening interferes with the heart’s ability to pump blood efficiently, potentially leading to heart failure if left untreated. A preliminary stage of heart disease with no visible symptoms, detectable only via laboratory tests.
Inclusion Criteria (Diagnosis Requirements)
- Left ventricular wall must be thickened.
- Measured via echocardiography (2D or M-mode).
- Left ventricular thickness ≥6 mm indicates subclinical HCM.
Exclusion Criteria (Conditions to Rule Out)
- Systemic Hypertension: Ensure normal blood pressure (high BP can cause wall thickening).
- Other Causes of Myocardial Thickness: e.g., natural thickening in athletes or other diseases.
- History Exclusion:
- Congestive Heart Failure (fluid buildup due to heart weakness).
- Arterial Thromboembolism (artery blockage by clots).
- Severe LV Outflow Obstruction (critical blood flow blockage).
Sirolimus Tablet for Cat Dosage & Administration
Recommended Rapamycin Dosage for Cats
- Administer 0.3 mg/kg of body weight orally once weekly.
How to Administer
- Give the tablet whole—do not cut, crush, or chew.
- Always administer with food.

Rapamycin Dosage for Cats
| Rapamycin Dosage for Cats : THE RAJASTHAN EXPRESS | |||
| Body Weight (kg) | 0.4 mg Tablet (Orange) | 1.2 mg Tablet (Blue) | 2.4 mg Tablet (White) |
|---|---|---|---|
| 2.5 – 3.2 kg | 2 | 0 | 0 |
| 3.3 – 4.8 kg | 0 | 1 | 0 |
| 4.9 – 6.4 kg | 1 | 1 | 0 |
| 6.5 – 9.6 kg | 0 | 0 | 1 |
| > 9.6 kg | 0 | 1 | 1 |
| Rapamycin Dosage for Cats : THE RAJASTHAN EXPRESS | |||
Sirolimus Tablet for Cat Side Effects & Contraindications
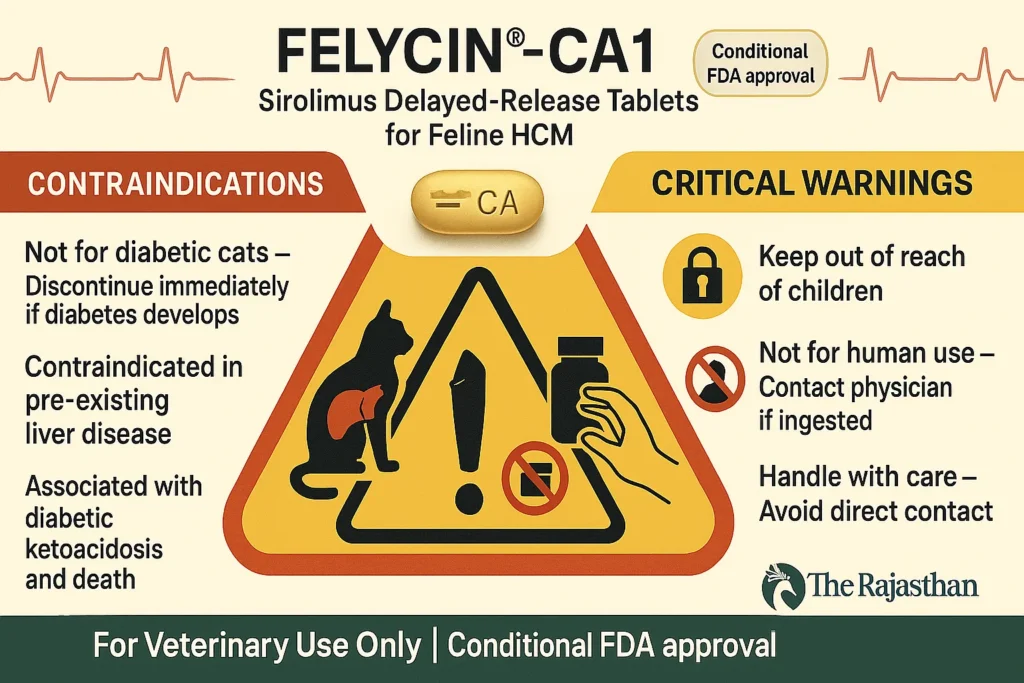
Critical Contraindications
- ❌ Do NOT use in diabetic cats.
- ❌ Discontinue immediately if diabetes develops during treatment.
- ❌ Avoid in cats with pre-existing liver disease.
Risk of Diabetic Ketoacidosis (DKA)
- Using FELYCIN®-CA1 in diabetic cats may trigger life-threatening Diabetic Ketoacidosis (DKA).
Diabetic Ketoacidosis (DKA) in Cats
What Is DKA?
A metabolic emergency caused by insulin deficiency/resistance, disrupting glucose metabolism. Cells cannot use glucose for energy, forcing the body to break down fat into ketone bodies.
Hormonal Role in Glucose Balance
- Glucagon: Secreted by pancreatic α-cells → raises blood glucose.
- Insulin: Secreted by pancreatic β-cells → lowers blood glucose.
Imbalance (e.g., insulin deficiency) causes diabetes.
Consequences of DKA
Excess ketones disrupt acid-base balance (pH), leading to:
- Dehydration
- Electrolyte imbalances (e.g., potassium/phosphorus deficiency)
- Organ dysfunction (heart, brain, kidneys).
DKA Treatment Protocol
- IV Fluid Therapy: Rehydrate and improve circulation.
- Regular/Short-Acting Insulin: Regulate blood glucose.
- Electrolyte Supplementation: Restore potassium/phosphorus.
- Continuous Monitoring:
- Blood glucose
- Ketone levels
- Acid-base status (pH)
Sirolimus Tablet for Cat Side Effects & Safety Warnings
Critical User Safety Warnings
- 🚫 Not for human use – Keep out of children’s reach.
- 🚫 If accidentally ingested by humans, seek immediate medical attention.
Handling & Administration Precautions
- ❌ Do NOT administer to pregnant or lactating cats.
- ❌ Use cautiously in cats with known Sirolimus allergy.
- ✅ Always store tablets in original packaging – Remove from blister pack ONLY at dosing time.
- ❌ If a cat refuses the tablet, destroy it immediately.
- 🧤 Wear gloves when cleaning vomit/saliva/tablet residue → Wash hands thoroughly afterward.
Note: The enteric coating protects against direct Sirolimus exposure during normal use. Chewing, vomiting, or tablet damage compromises this barrier.
Drug Interactions & Special Risks
Sirolimus and Other Medications
Sirolimus is metabolized by enzymes/proteins (CYP3A4, P-glycoprotein). High-risk interactions with:
- Calcium channel blockers (blood pressure meds)
- Amiodarone (heart rhythm regulator)
- Azole antifungals (e.g., Ketoconazole)
- Cyclosporine (immunosuppressant)
→ May cause toxic Sirolimus buildup.
High-Risk Cats: MDR1 Gene Mutation
Cats with MDR1 gene mutations have reduced drug-elimination capacity → Higher sensitivity to FELYCIN®-CA1. Use extreme caution with:
- Eprinomectin
- Emodepside
Effect on Vaccinations
FELYCIN®-CA1 may suppress immune response to vaccines:
- 🟢 Rabies (killed virus): No significant impact observed.
- 🔴 Other vaccines: Efficacy uncertain for:
- FHV-1 (Feline Herpes)
- FCV (Calicivirus)
- FPV (Panleukopenia)
- FeLV (Leukemia)
Sirolimus Tablet for Cat Side Effects: Clinical Study Findings
Pilot Field Study Overview
- *180-day controlled trial with 43 subclinical HCM cats:*
| Sirolimus Tablet for Cat Side Effects: Pilot Field Study Overview | ||
| Group | Dose | Cats (n) |
|---|---|---|
| Group 1 | 0.3 mg/kg/week | 15 |
| Group 2 | 0.6 mg/kg/week | 15 |
| Placebo | None | 13 |
| Sirolimus Tablet for Cat Side Effects | ||
Cardiac Side Effects
Most common in medicated cats (linked to HCM progression):
- ⚠️ Arrhythmia (irregular heartbeat)
- ⚠️ Congestive Heart Failure (fluid buildup)
- ⚠️ Syncope (sudden fainting)
- ⚠️ Pericardial Effusion (fluid around heart)
Critical High-Dose Cases (0.6 mg/kg):
3 cats experienced:
- Sudden death or severe heart failure
- 2 had pre-existing advanced heart disease
- 1 showed elevated biomarker NTproBNP (1344 pmol/L; normal <100) indicating high progression risk.
Note: Causality not confirmed due to small sample size and variable HCM progression.
Non-Cardiac Side Effects
Common across treatment groups:
- Lethargy
- Vomiting
- Diarrhea
- Inappetence
Diabetes Mellitus Risk
- One cat (0.3 mg/kg group) developed:
- Hypercholesterolemia
- Hyperglycemia
- Glucosuria
- UTI symptoms
→ Progressed to fatal Diabetic Ketoacidosis (DKA) → Cardiac arrest.
Liver Disease Complications
- Pre-existing elevated liver enzymes (ALP/ALT/AST):
- Worsened anorexia
- Further enzyme elevation
- Icterus (jaundice)
→ Euthanized 4 months post-study due to irreversible decline.
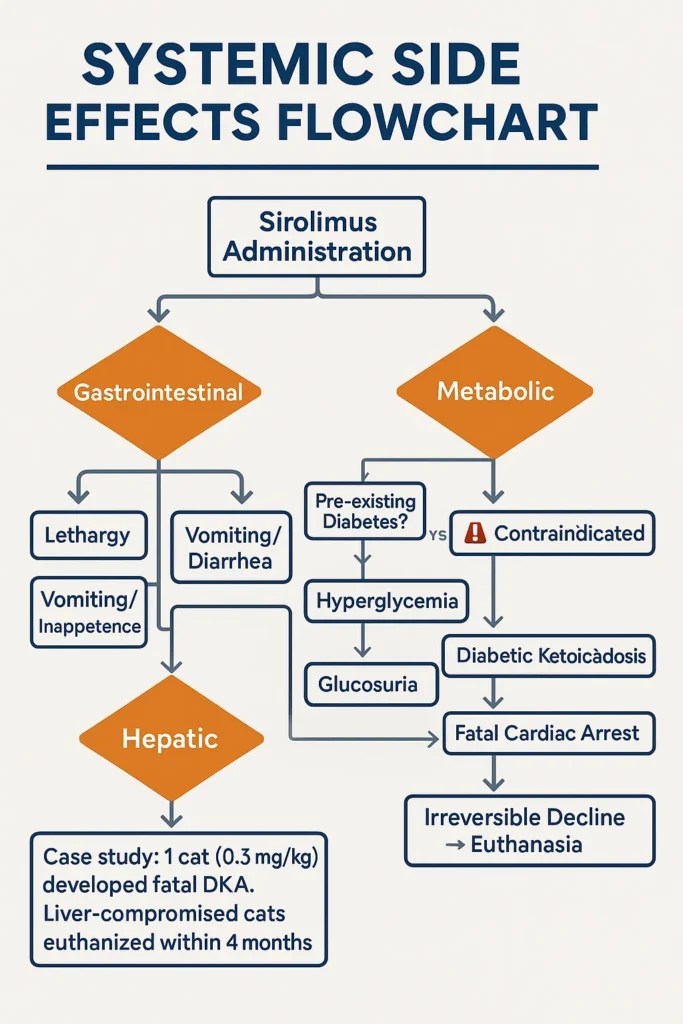
Conclusion: Managing Risks with Sirolimus for Cats
Key Safety Recommendations
- ❗ Contraindicated in cats with:
- Pre-existing diabetes
- Liver disease
- ⚠️ High-risk patients:
- Advanced HCM (NTproBNP >100 pmol/L)
- MDR1 gene mutation
- ✅ Mandatory protocols:
- Weekly cardiac monitoring
- Blood glucose checks
- Liver enzyme panels every 4-6 weeks
- 🚨 Immediate discontinuation if:
- Hyperglycemia symptoms appear
- Appetite loss persists >48 hrs
*FELYCIN®-CA1 requires vigilant risk-benefit assessment—reserve for early-stage HCM with no comorbidities.*
Contact Information
Report adverse events or seek assistance:
- 🏥 PRN Pharmacal: 1-800-874-9764
- ⚕️ FDA Veterinary Adverse Events:
- ☎ 1-888-FDA-VETS
- 🌐 www.fda.gov/reportanimalae
Clinical Pharmacology of Felycin-CA1 Tablets
Mode of Action: How Sirolimus Works in Cats
Sirolimus is an immunosuppressant drug primarily used in human organ transplants to prevent antibody rejection. It inhibits the protein complex mTORC1 (mammalian Target of Rapamycin Complex 1), which regulates:
- Cell growth
- Nutrient response
Observed effects in studies:
- ↓ Cardiac hypertrophy (abnormal heart muscle thickening)
- ↑ Autophagy (cellular self-cleaning via lysosomes)
- ↓ Oxidative stress
- ↓ Inflammatory responses
→ Sirolimus improves cardiac outcomes in cats.
Sirolimus Pharmacokinetics in Cats
Drug Absorption, Distribution & Elimination
Pharmacokinetics describes how a drug is:
- Absorbed
- Distributed
- Metabolized
- Eliminated
*24-week study in healthy cats (once-weekly dosing):*
Key Findings :
- Saturation Effect:
- Higher doses DO NOT increase absorption → Body absorbs only up to a threshold.
- *Implication: Exceeding 0.3 mg/kg/week provides no added benefit.*
- Non-Linear Kinetics:
- Drug effects vary over time → Repeated dosing alters response patterns.
Sirolimus Pharmacokinetics at 0.38 mg/kg Dose
Drug Accumulation Findings
*147-day observation in cats revealed significant drug accumulation:*
| Sirolimus Pharmacokinetics at 0.38 mg/kg Dose: Drug Accumulation Findings | |
| Parameter | Increase vs. Baseline |
|---|---|
| Cmax (Peak blood concentration) | 1.33× |
| AUClast (Total drug exposure) | 1.62× |
| 147-day observation in cats revealed significant drug accumulation. | |
| Sirolimus Pharmacokinetics at 0.38 mg/kg Dose: Key Pharmacokinetic Parameters | ||
| Parameter | Value (±SD) | Definition |
|---|---|---|
| AUClast | 288 ± 198 h·ng/mL | Total drug effect while measurable |
| Cmax | 22.0 ± 15.8 ng/mL | Maximum blood concentration |
| t½ | 71.8 ± 42.0 hrs | Half-life (50% drug elimination time) |
| Tmax | 1.50 hrs (1–12 hrs) | Time to reach peak concentration |
| Key parameters help characterize drug behavior and clearance. | ||
Conditional FDA Approval: Reasonable Expectation of Effectiveness
What This Means
FELYCIN®-CA1 holds conditional FDA approval (March 2025), not full approval. This indicates:
- ✅ Preliminary studies show therapeutic promise for feline HCM.
- ⚠️ Large-scale validation required for conclusive evidence.
Evidence Supporting Sirolimus Effectiveness in Cats
Published Preclinical Studies
- Rat Models:
- ↓ Left Ventricular Hypertrophy (LVH)
- ↑ Cardiac function improvement
- Human Transplant Patients:
- Enhanced cardiac remodeling post-surgery
| Pilot Field Study (43 Cats with Subclinical HCM): Study Design | ||
| Group | Dose | Cats (n) |
|---|---|---|
| Group 1 | 0.3 mg/kg/week | 15 |
| Group 2 | 0.6 mg/kg/week | 15 |
| Placebo | None | 13 |
| Felycin-CA1 Tablets: Sirolimus Tablet for Cats Approved for Subclinical HCM | ||
Results (180 Days):
- Withdrawals: 6 cats (5 high-dose, 1 placebo) due to:
- Worsening heart disease
- Accidental death
- Owner decision
| Myocardial Wall Thickness (MWT) Change Over 180 Days | ||
| Group | MWT Change | Outcome |
|---|---|---|
| 0.3 mg/kg | ↓ 0.17 mm | ✅ Improvement |
| Placebo | ↑ 0.94 mm | ❌ Worsening |
| 0.6 mg/kg | ↑ 0.50 mm | ❌ Worsening |
| Optimal results at 0.3 mg/kg with statistically significant improvement (p < 0.05) at Day 60/180. | ||
Conclusions & Future Needs
- FELYCIN®-CA1 demonstrates potential efficacy in:
- Reducing cardiac muscle thickness
- Improving heart structure
- ❗ Mandatory next steps:
- Large-scale, long-term clinical trials
- Ongoing safety monitoring
Source By : FDA & DAILYMED
Felycin®-CA1 (Sirolimus) has been conditionally approved by the U.S. Food and Drug Administration (FDA) on March 14, 2025, for the treatment of Subclinical Hypertrophic Cardiomyopathy (HCM) in cats. This makes it the first FDA-approved drug globally for managing this specific feline heart condition.
THE RAJASTHAN EXPRESS
Frequently Asked Questions About Felycin-CA1 (Sirolimus for Cats)
Can Felycin-CA1 be used in cats with early-stage kidney disease or hyperthyroidism alongside HCM?
No, and it is contraindicated.
- Safety Not Evaluated: The drug’s safety and effectiveness have not been studied in cats with CKD, hyperthyroidism, or other systemic illnesses.
- Exclusion Criteria: Clinical trials explicitly excluded cats with comorbidities like CKD or hyperthyroidism.
- Risk of Toxicity: Sirolimus is metabolized in the liver; impaired kidney or liver function may increase toxicity (e.g., elevated liver enzymes, diabetic ketoacidosis).
- Recommendation: Screen for comorbidities (creatinine, SDMA, T4) before prescribing. Discontinue if such conditions develop during treatment.
What does FDA conditional approval mean for Felycin-CA1’s availability and long-term safety?
- Basis: Approval (March 14, 2025) was based on safety and “reasonable expectation of effectiveness” from a 43-cat pilot study.
- Requirements: TriviumVet must complete the HALT-HCM study (n=300 cats) by 2028 for full FDA approval.
- Availability: Launched July 2025 for prescription use. Tablets come in 0.4 mg, 1.2 mg, and 2.4 mg strengths.
- Safety Monitoring: Long-term risks (diabetes, liver toxicity) are still under study. Adverse events must be reported to the FDA.
How to dose Felycin-CA1 for cats under 2.5 kg since tablets can’t be split?
- Tablets cannot be split or crushed due to their delayed-release coating.
- Lowest Tablet Strength: The 0.4 mg tablet (orange) may be used for cats ≥1.25 kg at 0.32 mg/kg (within 10% of the 0.3 mg/kg target dose).
- Compounding Not Recommended: Compounded sirolimus lacks safety and may not replicate Felycin’s release profile.
- Alternative: Delay treatment until the cat reaches 2.5 kg or consult PRN Pharmacal (1-800-874-9764).
Can Felycin-CA1 be given with other HCM drugs like beta-blockers or clopidogrel?
- Beta-blockers/antiarrhythmics: Contraindicated. Drugs like diltiazem and amiodarone inhibit CYP3A4/P-gp, increasing sirolimus toxicity.
- Clopidogrel: No reported interactions, but safety hasn’t been tested. Monitor for bleeding or lethargy.
- General Rule: Avoid multiple drugs unless absolutely necessary. Data on combination safety is limited.
What monitoring protocol is recommended for diabetes risk with Felycin-CA1?
- Baseline: Blood glucose, cholesterol, and urinalysis before treatment.
- Monthly: Monitor glucose levels and weight.
- Stop treatment immediately if: Blood glucose >180 mg/dL, glucosuria appears, or appetite loss persists >48 hours.
- High-risk cats: Screen for obesity or elevated fructosamine before starting therapy.
Does Felycin-CA1 suppress immune function in cats long-term despite low dosing?
- Rabies vaccination: Response to killed rabies vaccine remains intact.
- Other vaccines: Live vaccines (e.g., FHV-1, FCV, FPV) may be less effective — avoid use during treatment.
- Trial criteria: Cats with chronic viral infections (e.g., FIV, FeLV) were excluded from studies.
Do certain cat breeds (Maine Coons, Ragdolls) respond better to Felycin-CA1?
- No breed-specific response data exists.
- Higher HCM risk: Maine Coons, Ragdolls, and Sphynx cats have a higher incidence of HCM but were not analyzed separately in the pilot study.
- Ongoing research: The HALT-HCM Study (2025–2028) may provide breed-specific insights.
Can Felycin-CA1 reverse existing heart damage or only prevent progression?
- It prevents progression but does not reverse damage.
- Pilot Study Result: At 0.3 mg/kg, cats showed a 0.17 mm reduction in wall thickness over 180 days; placebo cats worsened by +0.94 mm.
- No effect on scarring: There’s no evidence of reversal of fibrosis or improved diastolic function.
- Goal: Delay onset of congestive heart failure or sudden death in subclinical HCM.
Is compounded rapamycin as effective as Felycin-CA1 for feline HCM?
- No — compounded versions are unsafe and not equivalent.
- Formulation differences: Compounded sirolimus lacks delayed-release coating, risking toxicity or underdosing.
- FDA violations: Petspan was cited for unproven safety claims regarding compounded sirolimus.
- Legal action: TriviumVet issued cease-and-desist notices against illegal compounders.
Can anti-aging rapamycin doses prevent HCM in high-risk cats?
- No studies support this use.
- FDA indication: Only for diagnosed subclinical HCM (wall thickness ≥6 mm).
- Theory vs. practice: While mTOR inhibition may slow aging, there’s no data in cats to support prophylactic dosing.
- Risks: Unnecessary dosing can lead to diabetes or liver toxicity.
What is the cost of Felycin-CA1 treatment per year for an average cat?
- Cost not officially published.
- Estimate: A 4 kg cat needs 1.2 mg/week (1 blue tablet).
- Annual supply: 52 tablets ≈ 4.3 cartons (12 tablets/carton).
- Estimated cost: $800–$1,200 per year (based on specialty veterinary drug pricing).
- Contact: For actual pricing, call PRN Pharmacal: 1-800-874-9764.


